Introduction: Golcadomide is a novel, oral, small-molecule CELMoD ® agent that co-opts cereblon to induce targeted degradation of the transcription factors Ikaros/Aiolos, which are crucial to B-cell malignancy development. We previously reported promising efficacy with a predictable and manageable safety profile of golcadomide in patients with relapsed/refractory (R/R) non-Hodgkin lymphoma (Michot et al. ICML 2023, presentation #90).
Methods: CC-99282-NHL-001 (NCT03930953) is a 2-part, multicenter, first-in-human study with dose escalation (Part A) of golcadomide monotherapy and expansion (Part B) ± combination partners. Patients with R/R diffuse large B-cell lymphoma (DLBCL) or R/R follicular lymphoma and disease progression after ≥ 2 lines of therapy or transplant-ineligible patients with R/R DLBCL after ≥ 1 line of therapy were included. Following dose exploration in Part A, golcadomide was administered at 0.2 or 0.4 mg alone or with rituximab on 2 intermittent schedules in Part B. Total duration of treatment is up to 2 years. Here, we report efficacy and safety results for golcadomide + rituximab in patients with DLBCL treated in Part B expansion on a 14 days on/14 days off schedule. The efficacy-evaluable population consisted of patients completing ≥ 1 cycle of golcadomide (taking ≥ 75% of assigned doses) and having baseline and ≥ 1 post-baseline tumor assessment.
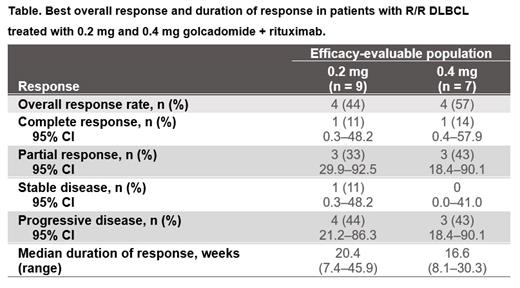
Results: As of May 10, 2023, 35 patients with DLBCL were enrolled and 34 received ≥ 1 dose of golcadomide or rituximab (safety population); 17 (49%) patients were ongoing and 17 (49%) had discontinued treatment, mostly due to progressive disease (n = 10, 29%). The median age was 60 years (range, 35-80). The median number of prior anticancer therapies was 4.5 (range, 1-11); 68% (n/N = 23/34) of patients had prior CAR T-cell therapy and 49% (n/N = 17/35) were refractory to their last regimen. Golcadomide median treatment duration was 8 weeks (range, 4-48) and median follow up was 15 weeks (range, 2-55). In the efficacy-evaluable population (n = 16), overall response rate (complete response [CR] + partial response) was 50% (n = 8), with CR occurring in 13% (n = 2) of patients. Median duration of response was 17.4 weeks (range, 7.4-45.9). Response data in the 0.2-mg and 0.4-mg golcadomide-treated groups are shown in the table. In the safety population (n = 34), neutropenia was the most common any-grade treatment-emergent adverse event (TEAE), occurring in 15 (44%) patients, all of which were grade 3/4. No febrile neutropenia occurred. The most common treatment-related adverse event (TRAE) for golcadomide was neutropenia (all grade 3/4), occurring in 14 (41%) patients, comprising 5/19 (26%) patients treated at the 0.2-mg and 9/15 (60%) patients treated at the 0.4-mg dose level. Granulocyte colony-stimulating factors were used in 14 (41%) patients. Six serious TRAEs related to golcadomide were reported (n = 1 each), all in the 0.4-mg group. One grade 5 TEAE occurred (tubulo-interstitial nephritis in the 0.2-mg group), which was reported to be due to progressive disease and considered unrelated to study treatment. TEAEs led to golcadomide discontinuation in 2 (6%) patients (1 at each dose level) and rituximab discontinuation in 5 (15%) patients. Similar pharmacokinetic (PK) exposures of golcadomide were observed when golcadomide was dosed alone and in combination with rituximab, indicating no apparent impact of rituximab on golcadomide PK. Analysis of circulating tumor DNA data is underway and will be presented.
Conclusions: Golcadomide oral therapy combined with rituximab showed promising efficacy in heavily pretreated patients with R/R DLBCL, including 1 durable response over 300 days. Golcadomide can be safely combined with rituximab, and the combination showed a safety profile similar to that previously reported for golcadomide monotherapy. This study is ongoing, with continued enrolment in the monotherapy and golcadomide + rituximab combination expansion cohorts.
Disclosures
Chavez:Genmab: Consultancy; Merck: Research Funding; Lilly: Honoraria, Speakers Bureau; ADC Therapetics: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Adicet: Consultancy; TG Therapeutics: Consultancy; Cellectar: Consultancy; Genentech, Inc: Consultancy; AstraZeneca: Consultancy, Research Funding; Epizyme: Honoraria; BeiGene: Honoraria, Speakers Bureau; Adaptive: Research Funding; Janssen: Research Funding; Genmab, ADC Therapetics, Kite/Gilead, BMS, Novartis, Adicet, TG Therapeutics, Cellectar, Genentech, Inc., AstraZeneca: Consultancy; Merck, AstraZeneca, Adaptive, Janssen: Research Funding; Lilly, Epizyme, BeiGene: Honoraria. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; AbbVie: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria. Morschhauser:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy; Genmab: Consultancy; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cartron:BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Emercell: Consultancy; Roche: Consultancy, Honoraria; Gilead: Honoraria; Janssen: Honoraria; MabQi: Consultancy; MedxCell: Consultancy; Novartis: Honoraria; Ownards Therapeutics: Consultancy; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; MedxCell, Ownards Therapeutics, MabQi, Emercell, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Consultancy; Jansen, Gilead, Novartis, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Honoraria. Joergensen:Incyte: Consultancy; Gilead: Consultancy; Genmab: Consultancy; AstraZeneca: Consultancy; SOBI: Consultancy; Janssen: Consultancy; Roche: Consultancy; Orion: Consultancy; Abbvie: Consultancy. Bachy:Pfizer: Honoraria, Other: Personal Fees; Takeda: Honoraria; Novartis: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Incyte: Honoraria; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Amgen: Research Funding; Roche: Consultancy, Honoraria; Kite, a Gilead Company: Honoraria, Other: Personal Fees. Bories:AbbVie: Membership on an entity's Board of Directors or advisory committees; Kite Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Patah:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. de Moucheron:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Carrancio:BMS: Current Employment, Current equity holder in publicly-traded company. Zheng:Bristol Myers Squibb: Current Employment; Janssen: Ended employment in the past 24 months. Mei:Bristol Myers Squibb: Current Employment. Pourdehnad:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal